2021. July 9.
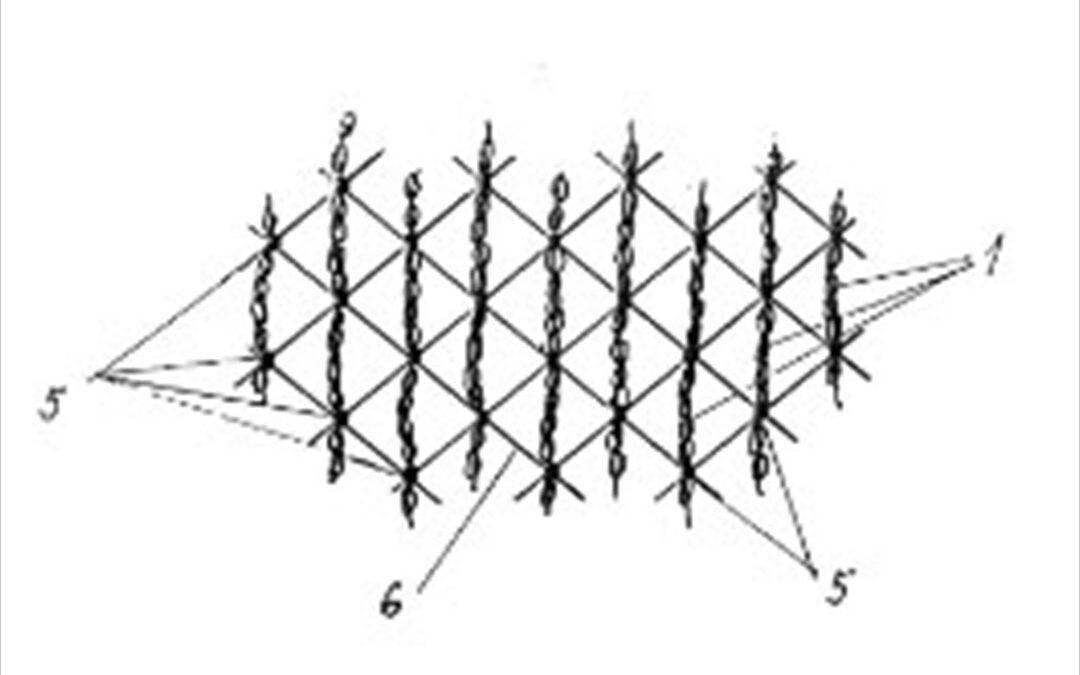
This invention relates to a biofilm carrier made of structured yarns (1) comprising filaments (2), where each individual filament (2) constituting the central yarn (1) forms a continuous fibre running over the entire length of the yarn (1) and filaments (2) are fixed to each other by anchor points (3) along the length of the yarn (1), and structured yarns (1) are fixed at fixing points (5) of a mesh structure (6), and each structured yarn (1) forms a loop (4) between two adjacent fixing points (5), the arc length (T) of which is greater than the distance (t) of the two fixing points (5).
2021. June 3.
The invention relates to a combined, solid, oral pharmaceutical composition that is usable for diminishing or protecting against the gastro-intestinal side effects of non-steroidal anti-inflammatory agents orally administered in a therapy, which contains capsaicinoid active agent in a small dose, one or more non-steroidal anti-inflammatory active agent, and one or more usual pharmaceutical auxiliary materials, characterized by that the composition comprises the active agent and other ingredients in a vehicle system which ensures the small concentration, uniform distribution and stability of the active agent on the mucous membrane.
2021. June 3.
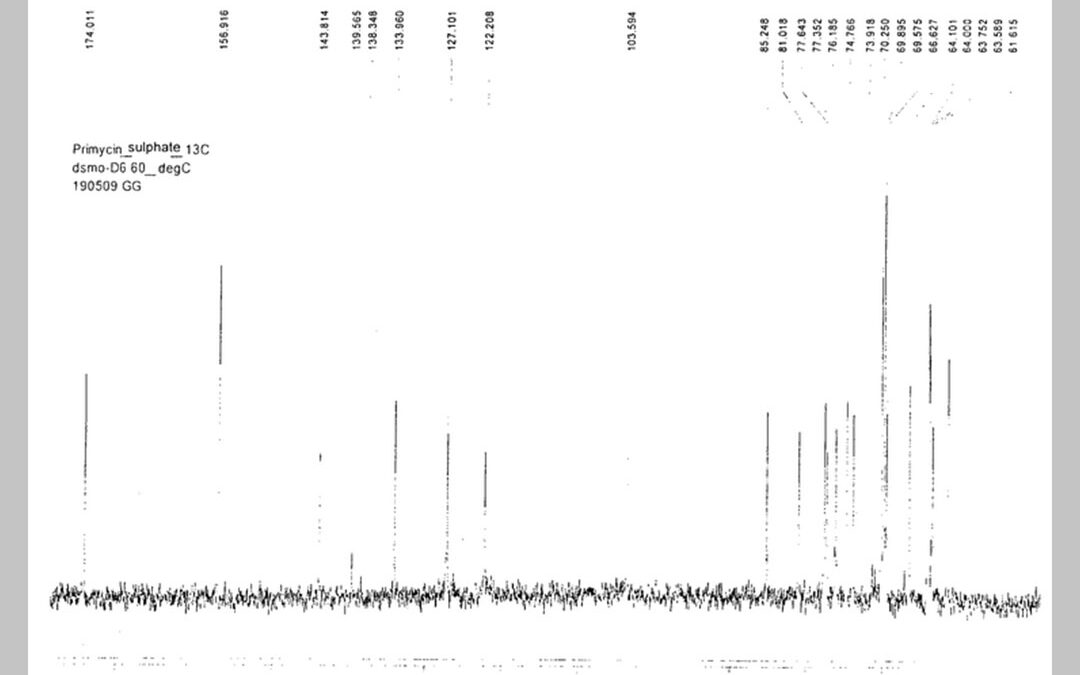
A process is disclosed for the production of primycin, components, precursors and metabolites thereof, wherein Saccharomonospora azurea, its variants or mutants are fermented in a medium and after reaching the appropriate concentration, the primycin, primycin components, precursors and metabolites are extracted with known methods or with a combination of known methods. In the process, Saccharomonospora azurea, its variants or mutants are used to produce primycin, primycin components, primycin precursors and metabolites.
2021. June 3.
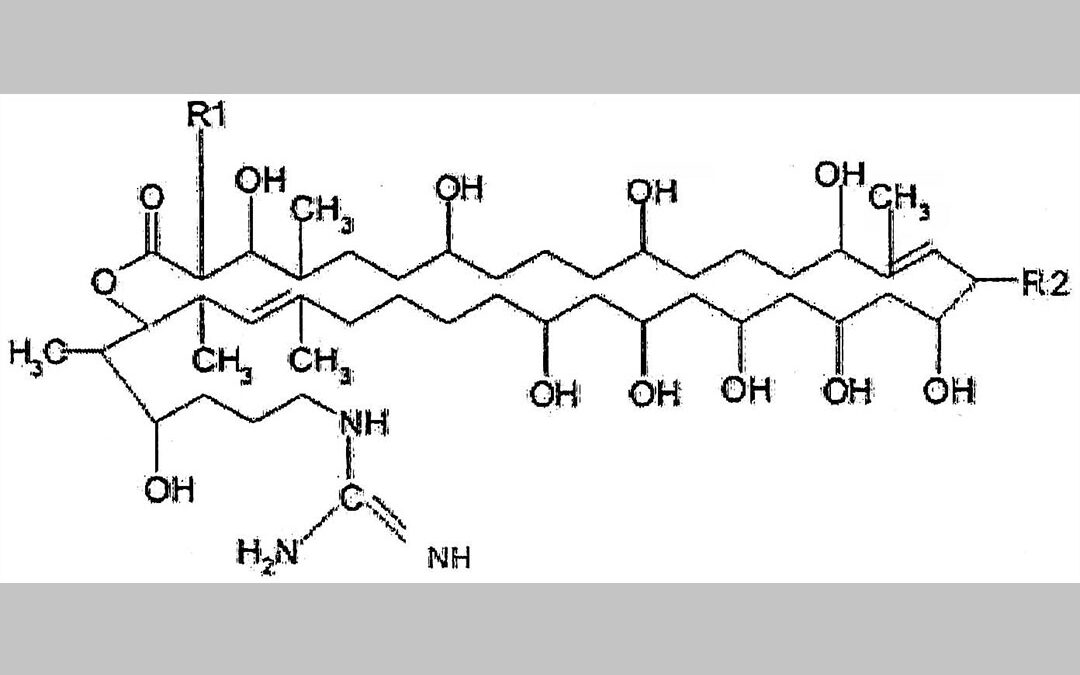
The invention relates to primycin or a primycin component or a combination of primycin components as an active substance, for use in the treatment or prevention of infections caused by methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant coagulase-negative Staphylococcus sp. (MRCoNS), vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus (VISA and VRSA), mupirocin-resistant Staphylococcus sp, vancomycin-resistant Enterococci (VRE) or primycin-resistant Streptococcus pneumoniae strains.

2021. May 24.
The present invention relates to a combination of three natural active ingredients, and to the topically applied preparation thereof.









